Jump to a lesson's notes
Unit 2
2.1 - Properties of Matter
2.2 - Mixtures
2.3 - Elements and Compounds
2.4 - Chemical Reactions
Unit 3
3.1 - Using and Expressing Measurements
3.2 - Units of Measurement
3.3 - Solving Conversion Problems
Unit 5
5.1 - Revising the Atomic Model
5.2 - Electron Arrangement in Atoms
5.3 - Atomic Emission Spectra and the Quantum Mechanical Model
Unit 7
Unit 8
2.1
Properties of Matter
Anything with a uniform and definite composition is a substance.
Aluminum (Al) / Copper (Cu)
- Aluminum is more reflective
- Copper can scratch Aluminum due to being harder
- Copper conducts electricity much better and thus is used for wires
- They are both malleable and can be pounded into sheets
Hardness, Color, Conductivity, and Malleability are all physical attributes used in identifying substances.
States of matter, boiling/freezing points, and odors are all more broad identifiers.
Solids have definite shapes not contingent on a container
- Particles are firmly packed together
- Exceedingly hard to compress
Liquids take the shape of the container they are in, whether it be a cup or a riverbed
- Particles are still somewhat packed in but freely flowing
- Near impossible to compress
- Slightly expands when heated
- Fixed Volume
Gasses take the shape of its' container but will expand to fit the volume
- Particles are loosely packed
When a substance is melted, the attributes of the substance will change, but its' composition WILL NOT
The terms gas and vapor shouldn't be used interchangeably. A vapor is a gas derived from another state of matter at room temperature.
Intensive properties depend on the type of substance. Extensive properties vary by the amounts of the substance.
2.2
Mixtures
Heterogeneous Mixtures are not even distributions of the components (chicken noodle soup)
Homogenous Mixtures have a consistent quantity of each component (olive oil)
Homogenous Mixtures are also referred to as solutions
Phases are parts of samples with uniform composition and properties
Filtration is running a substance through another (coffee).
Filtration depends on the size of the particles of the given substance(s).
Distillation involves different substances boiled into vapor and is then recondensed into liquid (beer).
Distillation depends on the boiling points of the substances.
2.3
Elements and Compounds
Elements are the most basic building block of matter
Compounds are combinations of elements in fixed proportions
Elements cannot be broken down any further, whereas compounds can.
Heating may be used to break down compounds.
is a soft silver metal with explosive capabilities.
Chlorine (Cl) is a deadly poisonous yellow gas.
Chloride Cl) (the combination of the two) is simply table salt.
The Periodic Table was originally developed by a Swedish man named Jacob Berzelius, with evident roots in Latin (e.g. Gold (Au) = aurum)
A column on the Periodic Table is a Group.
A row on the Periodic Table is a Period.
Substances haved fixed composition, and mixtures vary in composition.
Water =
Sucrose =
2.4
Chemical Reactions
Chemical changes always have a change in composition. Physical changes never do so.
Burn, rot, rust, decompose, ferment, and explode are words use to describe chemical changes.
A substance's ability to undergo a specific chemical change is a chemical property.
Breaking off a chunk of coal is physical, lighting it ablaze and having it burn is chemical.
Possible indicators of chemical changes are changes in color and temperature, emission of gas, and formation of precipitate.
A precipitate is a solid derived from a liquid mixture that settles (cottage cheese curdling from milk)
However, you cannot be assured of chemical change even with any of these clues unless you test the composition of the product against its' reactants.
Chemical changes are strictly irreversible, physical changes may or may not be undone.
The mass of the product(s) = The mass of the reactant(s).
3.1
Using and Expressing Meaurements
In chemistry you will find very small and very large numbers.
Scientific Notation is a way to shorten these numbers 6,300,000 = 6.3 x 106
There are exponents (Elementary school level concept) and coefficients, being the numbers that aren't exponents.
94,700 = 9.47 x 104
Numbers larger than 10 will have positive exponents and shift to the left.
Numbers smaller than 10 will have negative exponents and will shift to the right.
Addition and subtraction in scientific notation requires that the exponents be the same.
5.4 x 103 + 8.0 x 102 = 5.4 x 103 + 0.80 x 103 (A shift to the left indicates negative)
(
5.4 + 0.80) x 103 = 6.2 x 103
To multiply in scientific notation, multiply the coefficients and add the exponents. LIke with addition, the exponents need to be identical.
(
8.0 x 10-2) x (7.0 x 10-5) = (8.0 x 7.0) x 10-2 + (-5)
56 x 10-7 = 5.6 x 10-6
Significant Figures are all digits of a measurement that are known, as well as the last one that is estimated.
0.8 m = 0.77 m = 0.772 m
The more precise your measuring tool, the more significant figures you'll have.
All non-zero numbers are automatically significant.
Zeroes in front of non-zeroes (also called Leading Zeroes) Are insignificant.
When zeroes are behind non-zeroes and there is a decimal somewhere in front, they are significant.
Numbers counted have infinite sig figs, while measured numbers have definite sig figs.
Accuracy refers to the closeness to the target.
Precision is the closeness between a set of repeated measurements, regardless of accuracy.
Error is found by subtracting the Accepted Value (the value established by rigorous testing and scientific consensus) from the Experimental Value (The value measured in a solitary experiment)
You dip a thermometer into boiling water, and it reads 99.1 °C. However, you know it is established to be 100 °C.
EX: 100° C (Accepted) - 99.1 °C (Experimental) = 0.9° C (Error)
3.2
Units of Measurement
3.3
Solving Conversion Problems
4.1
Atomic Structure
Atoms are the smallest part of an element that still holds the element's chemical properties.
The Greek philospher Democritus (ca. 370 BC) was the first person to surmise the existence of atoms. He was, however inaccurate in the belief that they were indivisible and indestructible. He also didn't use the Scientific Method to make these assertions.
John Dalton (1766-1864), an English chemistry teacher, would go on to confirm Democritus' musings of the existence of the small building blocks of matter.
Dalton's Atomic Theory states:
All atoms of a given element are the same
Two atoms pertaining to different elements are different
Atoms from different elements can combine in fixed ratios to create compounds
Atoms cannot be changed into other elements as a result of chemical reaction. They can only be separated, joined or rearranged in various ways.
Dalton receives more credit for the discovery of Atoms than Democritus 2000 years earlier, because Dalton could experiment and produce his assertions in real life. Democritus didn't experiment because the Scientific Method didn't yet exist.
4.2
Structure of the Nuclear Atom
The only thing John Dalton happened to miss is that atoms can in fact, be further divided. Although it certainly doesn't end well for anyone who does so without adequately backing away.
In 1897, Another Englishman, Physicist J. J. Thompson, had discovered the Electron. Thompson originally coined the negatively charged Subatomic Particles 'corpuscules'.
American Physicist Robert A. Milikan determined the charge of a single electron.
Atoms have no net electric charge. They are neutral.
You can't have a fraction of an electrical charge in atoms.
Eugen Goldstein while observing the same Cathode Ray which allowed Thompson to conclude that electrons existed, flipped the experiment to observe Protons.
James Chadwick discovered the Neutron.
Ernest Rutherford from New Zealand, in an effort to confirm the Plum-Pudding model, shoots a beam of double-positive charged helium atoms (The atoms are missing two electrons) at a sheet of gold foil.
Based on Rutherford's results, he concluded that atoms are mostly empty space, as opposed to bunched together as J. J. Thompson previously stated in the Plum-pudding model.
To illustrate the emptiness around the nucleus, If atoms were the size of football fields, nuclei would be marbles.
The new model was called Rutherford's atomic model and he received a Nobel Prize in 1908 for his discovery.
4.3
Distinguishing Among Atoms
The amount of protons change the element and the Atomic Number.
The Atomic Number (the bottom number) is the amount of protons in an element.
Atoms have to be electrically neutral. They need to have the same amount of electrons as they do protons.
The Mass Number (the top number) is dictated by the total amount of the protons (-) and neutrons, seeing as how electrons are so tiny and light their mass hardly exists.
Atomic numbers use subscript.
Mass numbers use superscript.
You can read an element in tandem with its Mass Number; Gold-197 / Au-197
Elements that have more or less neutrons than electrons/protons, are called isotopes.
An Atomic Mass Unit is equivalent to 1/12 of the mass of a Carbon-12 atom.
An element's Atomic Mass is the average mass across multiple samples.
If Copper has an atomic mass of 63.546 amu, then Copper-63 is more abundant than Copper-65.
5.1
Revising the Atomic Model
Limitations in Rutherford's Model included only showing a few basic properties of atoms, and not explaining chemical properties of given elements.
Niels Bohr, from Denmark, devised a new atomic model in 1913 that includes the new discovery of change in atoms when they absorb or emit light.
Bohr proposed that electrons orbit around the nucleus in fixed paths.
A quantum refers to the amount of energy needed for an electron to jump between Energy Levels (The different levels of orbits)
Erwin Schrodinger developed a set of mathematical equations called the Quantum Mechanical Model, This model determines the probability of where an electron might be.
An Atomic Orbital represents a visual space where one might find an electron.
5.2
Electron Arrangement in Atoms
5.3
Atomic Emission Spectra and the Quantum Mechanical Model
Electric current passing through Neon (Ne) gas allows for neon lighting.
Amplitude is a wave's height from zero to the crest.
Wavelength (λ) is the distance between the crests.
Frequency (v) is the amount of cycles to pass through a given point in a certain amount of time.
Formula for the speed of light; C (the speed of light) = λ (wavelength) multiplied by v (frequency) / c = λv
The SI unit of measurement for frequency is hertz (Hz).
Frequency and wavelength are inverse to each other.
All colors are represented in light. Red light has the lowest frequency.
The Electromagnetic Spectrum:
 Electromagnetic spectra differ from element to element in the ways they refract.
Light quanta are called photons.
Light inhibits both wave-like and particle-like properties.
Red light cannot bounce electrons off of other objects because the frequency doesn't meet the threshold for energy. With higher frequencies however, starting with green light, the electrons will not only begin to bounce off of surfaces, but if you increase the frequency further (i.e. purple light),
said electrons bounce off proportionally
faster. Electron microscopes work due to the light bouncing back into the lens.
The Heisenberg Uncertainty Principle states that you cannot know the position and the velocity of a particle simultaneously.
*obviously, this does not apply to any object visible to the naked eye.
Electromagnetic spectra differ from element to element in the ways they refract.
Light quanta are called photons.
Light inhibits both wave-like and particle-like properties.
Red light cannot bounce electrons off of other objects because the frequency doesn't meet the threshold for energy. With higher frequencies however, starting with green light, the electrons will not only begin to bounce off of surfaces, but if you increase the frequency further (i.e. purple light),
said electrons bounce off proportionally
faster. Electron microscopes work due to the light bouncing back into the lens.
The Heisenberg Uncertainty Principle states that you cannot know the position and the velocity of a particle simultaneously.
*obviously, this does not apply to any object visible to the naked eye.
6.1
Organizing the Elements
Dmitri Mendelev made the first periodic table. He arranged them in increasing order of atomic mass.
Mendelev's Periodic Table
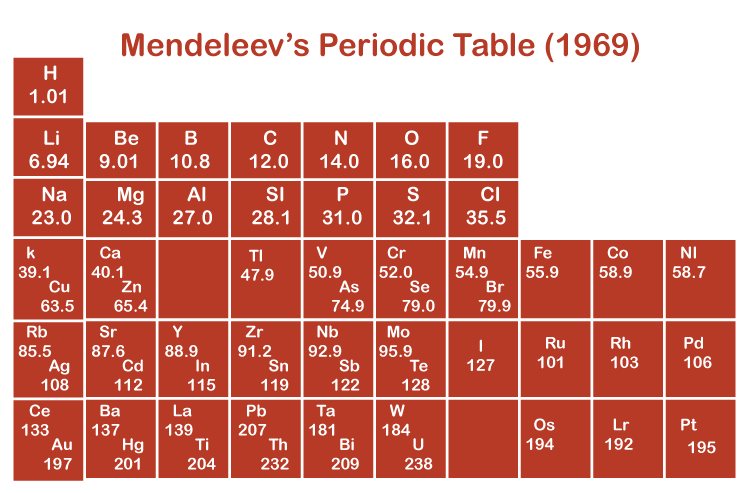 Even finding out about many elements in the 1960's, he had some unknowns. These unknown elements were discovered years later.
Even finding out about many elements in the 1960's, he had some unknowns. These unknown elements were discovered years later.
The Modern Periodic Table
 This table is much more complete and organized, elements being sorted in rows (periods) by energy levels, and
the columns (groups) sort by certain sets of shared chemical and/or physical properties.
The three primary types of elements are metals (80%), non-metals, and metalloids.
Most metals are malleable and can be pounded into sheets.
Nonmetals are gases at room temperature. The few exceptions are , and which are normally solids, and which is liquid. While metallic properties are rather *IRON-CLAD*, One blanket set of non-metallic properties is impossible to deduce given the variation of forms they can take on. But they tend to take on properties opposite of metals.
Metalloids hold the smallest presence on the Periodic Table, and holds some properties of both metals and non-metals.
in itself, is bad as an electrical conductor, but you mix some into it, suddenly we have the basis for all motherboards and computer chips.
This table is much more complete and organized, elements being sorted in rows (periods) by energy levels, and
the columns (groups) sort by certain sets of shared chemical and/or physical properties.
The three primary types of elements are metals (80%), non-metals, and metalloids.
Most metals are malleable and can be pounded into sheets.
Nonmetals are gases at room temperature. The few exceptions are , and which are normally solids, and which is liquid. While metallic properties are rather *IRON-CLAD*, One blanket set of non-metallic properties is impossible to deduce given the variation of forms they can take on. But they tend to take on properties opposite of metals.
Metalloids hold the smallest presence on the Periodic Table, and holds some properties of both metals and non-metals.
in itself, is bad as an electrical conductor, but you mix some into it, suddenly we have the basis for all motherboards and computer chips.
6.2
Classifying the Elements
Possible details shown on an entry on the Periodic Table, using Aluminum as an example:
 Elements with Black text are solids at room temperature, red means gas, and blue means liquid. Only two elements are liquid at room temp, being Mercury and Bromine.
Elements in gray denote that they do not naturally occur.
The elements in groups 1A through 7A are called Representative Elements, because they show the widest variety of physical and chemical properties.
The term 'inner transition metal' is rather misleading, given they're in far greater quantities than a lot of normal metals.
Elements with Black text are solids at room temperature, red means gas, and blue means liquid. Only two elements are liquid at room temp, being Mercury and Bromine.
Elements in gray denote that they do not naturally occur.
The elements in groups 1A through 7A are called Representative Elements, because they show the widest variety of physical and chemical properties.
The term 'inner transition metal' is rather misleading, given they're in far greater quantities than a lot of normal metals.
6.3
Periodic Trends
Atomic Radius is the size of the nucleus of a given atom. It is also half the distance between two identical nuclei when they are joined, measured in picometers (pm).
Further down (by groups) the periodic table you go, the larger the nuclei you will find for the respective elements' atoms. The size decreases as you move right (by period).
This is the trend for atomic size.
Net positive ions are called Cations and net negative ions are called Anions. Nonmetals tend to form anions, and metals tend to form cations.
The energy needed to remove an electron from an atom is Ionization Energy.
First Ionization Energy is the ionization energy needed for the first time an electron is removed from an atom. The energy requirement for first ionization energy increases left to right and decreases top to bottom. This is the inverse of the atomic size trend.
The first ionization energy trend is identical to the ionic size trend.
7.1
Ions
As you increase in groups, the amount of valence electrons increases (Group 1A has 1, Group 8A has 8, except for Helium).
Valence Electrons are the electrons found in the highest occupied energy level of an atom.
Cations are made when an atom loses one or more valence electrons.
Anions are made upon receiving one or more valence electrons.
7.2
Ionic Bonds & Ionic Compounds
Ionic Compounds are compounds composed of cations and anions, and are neutral (The total negative charge is equal to the total positive charge). Ionic Bonds are the electrostatic forces hold ions together in the compound.
A formula unit is the lowest whole-number ratio of ions in an ionic compound. The majority of ionic compounds are crystals at room temperature, are rather stable and have high melting points. Ionic compounds can conduct electricity when melted or dissolved in water. The Coordination Number refers to the amount of ions of opposite charge that surround the ion in the crystal.
Unit 2
Unit 3
Unit 5
Unit 7
Unit 8
2.1
Properties of Matter
Anything with a uniform and definite composition is a substance. Aluminum (Al) / Copper (Cu)
- Aluminum is more reflective
- Copper can scratch Aluminum due to being harder
- Copper conducts electricity much better and thus is used for wires
- They are both malleable and can be pounded into sheets
- Particles are firmly packed together
- Exceedingly hard to compress
- Particles are still somewhat packed in but freely flowing
- Near impossible to compress
- Slightly expands when heated
- Fixed Volume
- Particles are loosely packed
2.2
Mixtures
Heterogeneous Mixtures are not even distributions of the components (chicken noodle soup) Homogenous Mixtures have a consistent quantity of each component (olive oil) Homogenous Mixtures are also referred to as solutions Phases are parts of samples with uniform composition and properties Filtration is running a substance through another (coffee). Filtration depends on the size of the particles of the given substance(s). Distillation involves different substances boiled into vapor and is then recondensed into liquid (beer). Distillation depends on the boiling points of the substances.
2.3
Elements and Compounds
Elements are the most basic building block of matter Compounds are combinations of elements in fixed proportions Elements cannot be broken down any further, whereas compounds can. Heating may be used to break down compounds. is a soft silver metal with explosive capabilities. Chlorine (Cl) is a deadly poisonous yellow gas. Chloride Cl) (the combination of the two) is simply table salt. The Periodic Table was originally developed by a Swedish man named Jacob Berzelius, with evident roots in Latin (e.g. Gold (Au) = aurum) A column on the Periodic Table is a Group. A row on the Periodic Table is a Period. Substances haved fixed composition, and mixtures vary in composition. Water = Sucrose =
2.4
Chemical Reactions
Chemical changes always have a change in composition. Physical changes never do so. Burn, rot, rust, decompose, ferment, and explode are words use to describe chemical changes. A substance's ability to undergo a specific chemical change is a chemical property. Breaking off a chunk of coal is physical, lighting it ablaze and having it burn is chemical. Possible indicators of chemical changes are changes in color and temperature, emission of gas, and formation of precipitate. A precipitate is a solid derived from a liquid mixture that settles (cottage cheese curdling from milk) However, you cannot be assured of chemical change even with any of these clues unless you test the composition of the product against its' reactants. Chemical changes are strictly irreversible, physical changes may or may not be undone. The mass of the product(s) = The mass of the reactant(s).
3.1
Using and Expressing Meaurements
In chemistry you will find very small and very large numbers. Scientific Notation is a way to shorten these numbers 6,300,000 = 6.3 x 106 There are exponents (Elementary school level concept) and coefficients, being the numbers that aren't exponents. 94,700 = 9.47 x 104 Numbers larger than 10 will have positive exponents and shift to the left. Numbers smaller than 10 will have negative exponents and will shift to the right. Addition and subtraction in scientific notation requires that the exponents be the same. 5.4 x 103 + 8.0 x 102 = 5.4 x 103 + 0.80 x 103 (A shift to the left indicates negative) ( 5.4 + 0.80) x 103 = 6.2 x 103 To multiply in scientific notation, multiply the coefficients and add the exponents. LIke with addition, the exponents need to be identical. ( 8.0 x 10-2) x (7.0 x 10-5) = (8.0 x 7.0) x 10-2 + (-5) 56 x 10-7 = 5.6 x 10-6 Significant Figures are all digits of a measurement that are known, as well as the last one that is estimated. 0.8 m = 0.77 m = 0.772 m The more precise your measuring tool, the more significant figures you'll have. All non-zero numbers are automatically significant. Zeroes in front of non-zeroes (also called Leading Zeroes) Are insignificant. When zeroes are behind non-zeroes and there is a decimal somewhere in front, they are significant. Numbers counted have infinite sig figs, while measured numbers have definite sig figs. Accuracy refers to the closeness to the target. Precision is the closeness between a set of repeated measurements, regardless of accuracy. Error is found by subtracting the Accepted Value (the value established by rigorous testing and scientific consensus) from the Experimental Value (The value measured in a solitary experiment) You dip a thermometer into boiling water, and it reads 99.1 °C. However, you know it is established to be 100 °C. EX: 100° C (Accepted) - 99.1 °C (Experimental) = 0.9° C (Error)
3.2
Units of Measurement
3.3
Solving Conversion Problems
4.1
Atomic Structure
Atoms are the smallest part of an element that still holds the element's chemical properties. The Greek philospher Democritus (ca. 370 BC) was the first person to surmise the existence of atoms. He was, however inaccurate in the belief that they were indivisible and indestructible. He also didn't use the Scientific Method to make these assertions. John Dalton (1766-1864), an English chemistry teacher, would go on to confirm Democritus' musings of the existence of the small building blocks of matter. Dalton's Atomic Theory states: All atoms of a given element are the same Two atoms pertaining to different elements are different Atoms from different elements can combine in fixed ratios to create compounds Atoms cannot be changed into other elements as a result of chemical reaction. They can only be separated, joined or rearranged in various ways. Dalton receives more credit for the discovery of Atoms than Democritus 2000 years earlier, because Dalton could experiment and produce his assertions in real life. Democritus didn't experiment because the Scientific Method didn't yet exist.
4.2
Structure of the Nuclear Atom
The only thing John Dalton happened to miss is that atoms can in fact, be further divided. Although it certainly doesn't end well for anyone who does so without adequately backing away. In 1897, Another Englishman, Physicist J. J. Thompson, had discovered the Electron. Thompson originally coined the negatively charged Subatomic Particles 'corpuscules'. American Physicist Robert A. Milikan determined the charge of a single electron. Atoms have no net electric charge. They are neutral. You can't have a fraction of an electrical charge in atoms. Eugen Goldstein while observing the same Cathode Ray which allowed Thompson to conclude that electrons existed, flipped the experiment to observe Protons. James Chadwick discovered the Neutron. Ernest Rutherford from New Zealand, in an effort to confirm the Plum-Pudding model, shoots a beam of double-positive charged helium atoms (The atoms are missing two electrons) at a sheet of gold foil. Based on Rutherford's results, he concluded that atoms are mostly empty space, as opposed to bunched together as J. J. Thompson previously stated in the Plum-pudding model. To illustrate the emptiness around the nucleus, If atoms were the size of football fields, nuclei would be marbles. The new model was called Rutherford's atomic model and he received a Nobel Prize in 1908 for his discovery.
4.3
Distinguishing Among Atoms
The amount of protons change the element and the Atomic Number. The Atomic Number (the bottom number) is the amount of protons in an element. Atoms have to be electrically neutral. They need to have the same amount of electrons as they do protons. The Mass Number (the top number) is dictated by the total amount of the protons (-) and neutrons, seeing as how electrons are so tiny and light their mass hardly exists. Atomic numbers use subscript. Mass numbers use superscript. You can read an element in tandem with its Mass Number; Gold-197 / Au-197 Elements that have more or less neutrons than electrons/protons, are called isotopes. An Atomic Mass Unit is equivalent to 1/12 of the mass of a Carbon-12 atom. An element's Atomic Mass is the average mass across multiple samples. If Copper has an atomic mass of 63.546 amu, then Copper-63 is more abundant than Copper-65.
5.1
Revising the Atomic Model
Limitations in Rutherford's Model included only showing a few basic properties of atoms, and not explaining chemical properties of given elements. Niels Bohr, from Denmark, devised a new atomic model in 1913 that includes the new discovery of change in atoms when they absorb or emit light. Bohr proposed that electrons orbit around the nucleus in fixed paths. A quantum refers to the amount of energy needed for an electron to jump between Energy Levels (The different levels of orbits) Erwin Schrodinger developed a set of mathematical equations called the Quantum Mechanical Model, This model determines the probability of where an electron might be. An Atomic Orbital represents a visual space where one might find an electron.
5.2
Electron Arrangement in Atoms
5.3
Atomic Emission Spectra and the Quantum Mechanical Model
Electric current passing through Neon (Ne) gas allows for neon lighting.
Amplitude is a wave's height from zero to the crest.
Wavelength (λ) is the distance between the crests.
Frequency (v) is the amount of cycles to pass through a given point in a certain amount of time.
Formula for the speed of light; C (the speed of light) = λ (wavelength) multiplied by v (frequency) / c = λv
The SI unit of measurement for frequency is hertz (Hz).
Frequency and wavelength are inverse to each other.
All colors are represented in light. Red light has the lowest frequency.
The Electromagnetic Spectrum:
 Electromagnetic spectra differ from element to element in the ways they refract.
Light quanta are called photons.
Light inhibits both wave-like and particle-like properties.
Red light cannot bounce electrons off of other objects because the frequency doesn't meet the threshold for energy. With higher frequencies however, starting with green light, the electrons will not only begin to bounce off of surfaces, but if you increase the frequency further (i.e. purple light),
said electrons bounce off proportionally
faster. Electron microscopes work due to the light bouncing back into the lens.
The Heisenberg Uncertainty Principle states that you cannot know the position and the velocity of a particle simultaneously.
*obviously, this does not apply to any object visible to the naked eye.
Electromagnetic spectra differ from element to element in the ways they refract.
Light quanta are called photons.
Light inhibits both wave-like and particle-like properties.
Red light cannot bounce electrons off of other objects because the frequency doesn't meet the threshold for energy. With higher frequencies however, starting with green light, the electrons will not only begin to bounce off of surfaces, but if you increase the frequency further (i.e. purple light),
said electrons bounce off proportionally
faster. Electron microscopes work due to the light bouncing back into the lens.
The Heisenberg Uncertainty Principle states that you cannot know the position and the velocity of a particle simultaneously.
*obviously, this does not apply to any object visible to the naked eye.
6.1
Organizing the Elements
Dmitri Mendelev made the first periodic table. He arranged them in increasing order of atomic mass.
Mendelev's Periodic Table
 Even finding out about many elements in the 1960's, he had some unknowns. These unknown elements were discovered years later.
Even finding out about many elements in the 1960's, he had some unknowns. These unknown elements were discovered years later.
The Modern Periodic Table
 This table is much more complete and organized, elements being sorted in rows (periods) by energy levels, and
the columns (groups) sort by certain sets of shared chemical and/or physical properties.
The three primary types of elements are metals (80%), non-metals, and metalloids.
Most metals are malleable and can be pounded into sheets.
Nonmetals are gases at room temperature. The few exceptions are , and which are normally solids, and which is liquid. While metallic properties are rather *IRON-CLAD*, One blanket set of non-metallic properties is impossible to deduce given the variation of forms they can take on. But they tend to take on properties opposite of metals.
Metalloids hold the smallest presence on the Periodic Table, and holds some properties of both metals and non-metals.
in itself, is bad as an electrical conductor, but you mix some into it, suddenly we have the basis for all motherboards and computer chips.
This table is much more complete and organized, elements being sorted in rows (periods) by energy levels, and
the columns (groups) sort by certain sets of shared chemical and/or physical properties.
The three primary types of elements are metals (80%), non-metals, and metalloids.
Most metals are malleable and can be pounded into sheets.
Nonmetals are gases at room temperature. The few exceptions are , and which are normally solids, and which is liquid. While metallic properties are rather *IRON-CLAD*, One blanket set of non-metallic properties is impossible to deduce given the variation of forms they can take on. But they tend to take on properties opposite of metals.
Metalloids hold the smallest presence on the Periodic Table, and holds some properties of both metals and non-metals.
in itself, is bad as an electrical conductor, but you mix some into it, suddenly we have the basis for all motherboards and computer chips.
6.2
Classifying the Elements
Possible details shown on an entry on the Periodic Table, using Aluminum as an example:
 Elements with Black text are solids at room temperature, red means gas, and blue means liquid. Only two elements are liquid at room temp, being Mercury and Bromine.
Elements in gray denote that they do not naturally occur.
The elements in groups 1A through 7A are called Representative Elements, because they show the widest variety of physical and chemical properties.
The term 'inner transition metal' is rather misleading, given they're in far greater quantities than a lot of normal metals.
Elements with Black text are solids at room temperature, red means gas, and blue means liquid. Only two elements are liquid at room temp, being Mercury and Bromine.
Elements in gray denote that they do not naturally occur.
The elements in groups 1A through 7A are called Representative Elements, because they show the widest variety of physical and chemical properties.
The term 'inner transition metal' is rather misleading, given they're in far greater quantities than a lot of normal metals.
6.3
Periodic Trends
Atomic Radius is the size of the nucleus of a given atom. It is also half the distance between two identical nuclei when they are joined, measured in picometers (pm). Further down (by groups) the periodic table you go, the larger the nuclei you will find for the respective elements' atoms. The size decreases as you move right (by period). This is the trend for atomic size. Net positive ions are called Cations and net negative ions are called Anions. Nonmetals tend to form anions, and metals tend to form cations. The energy needed to remove an electron from an atom is Ionization Energy. First Ionization Energy is the ionization energy needed for the first time an electron is removed from an atom. The energy requirement for first ionization energy increases left to right and decreases top to bottom. This is the inverse of the atomic size trend. The first ionization energy trend is identical to the ionic size trend.
7.1
Ions
As you increase in groups, the amount of valence electrons increases (Group 1A has 1, Group 8A has 8, except for Helium). Valence Electrons are the electrons found in the highest occupied energy level of an atom. Cations are made when an atom loses one or more valence electrons. Anions are made upon receiving one or more valence electrons.
7.2
Ionic Bonds & Ionic Compounds
Ionic Compounds are compounds composed of cations and anions, and are neutral (The total negative charge is equal to the total positive charge). Ionic Bonds are the electrostatic forces hold ions together in the compound. A formula unit is the lowest whole-number ratio of ions in an ionic compound. The majority of ionic compounds are crystals at room temperature, are rather stable and have high melting points. Ionic compounds can conduct electricity when melted or dissolved in water. The Coordination Number refers to the amount of ions of opposite charge that surround the ion in the crystal.